Home > Parameters > Description of Parameters that we measure
Description of Parameters we measure
All gaseous and non-gaseous parameters and their detailed description.

Nitrogen Dioxide (NO2)
It primarily gets in the air from the burning of fuel, emissions from cars, trucks and buses, power plants, and off-road equipment.
Specifications we measure
| Range | Resolution | Technology | Sensor Module | |
| 50 PPM | 0.1 | Electrochemical | ES-NO2-4EC50 |
Guidelines
- WHO: 200μg/m3 (1-hr)
- OSHA: 5ppm (8-hr)
- US EPA: 53ppb (annual)
Health effects
- Irritates airways
- Longer exposures to NO2 may contribute to the development of asthma increase susceptibility to respiratory infections.

Ozone (o3)
is created by chemical reactions between oxides of nitrogen (NOx) and volatile organic compounds (VOC)
Specifications we measure
| Range | Resolution | Technology | Sensor Module | |
| 50 PPM | 0.01 | Electrochemical | ES-O3-4EC50 |
Guidelines
- OSHA: 0.1 ppm limit
- US EPA: 0.12 ppm limit
Health effects
- Eyes itch, burn, respiratory disorders, lowers our resistance to colds and pneumonia.
- Very harmful lifelong effects on the respiratory system

Hydrogen (H2)
Hydrogen gas (H₂) is primarily produced through industrial processes such as natural gas reforming, electrolysis of water, and biomass conversion. It is a colorless, odorless, and non-toxic gas under normal conditions.
Specifications we measure
| Range | Resolution | Technology | Sensor Module | |
| 2000 PPM | 1 | Electrochemical | ES-H2-4EC2000 |
Guidelines
- OSHA: No PEL for H₂, but oxygen levels must remain above 19.5%.
- US EPA: No specific NAAQS, but hydrogen levels should be below the Lower Explosive Limit (LEL) of 4% in air
Health effects
- Asphyxiation: Displaces oxygen in confined spaces, leading to dizziness, confusion, and unconsciousness.
- Flammability: Highly flammable, forming explosive mixtures (4-75% hydrogen in air).
- Breathing Issues: High concentrations may cause dizziness, chest tightness, and difficulty breathing.

Hydrogen Sulfide (H2S)
Produced by the breakdown of organic matter (e.g., sewage, oil refining, natural gas processing
Specifications we measure
| Range | Resolution | Technology | Sensor Module | |
| 200 PPM | 0.1 | Electrochemical | ES-H2S-4EC200 |
Guidelines
- OSHA:20 ppm over an 8-hour workday, with a 50 ppm short-term exposure limit (STEL) for 10 minutes
- US EPA: Not established for H₂S by the US EPA, but recommended limit for ambient air is typically 0.03 ppm (30-minute average).
Health effects
- Short Term: Eye irritation, sore throat, cough, and headaches.
- Long Term :Respiratory distress, loss of consciousness, paralysis, and death.

Ammonia (NH₃)
Industrial production through the Haber-Bosch process, used in fertilizers, refrigeration, and chemical production
Specifications we measure
| Range | Resolution | Technology | Sensor Module | |
| 200 PPM | 1 | Electrochemical | ES-NH3-4EC200 |
Guidelines
- OSHA:50 ppm over an 8-hour workday
- US EPA: No specific NAAQS for ammonia, but guidelines recommend ambient concentrations not exceeding 0.1 ppm
Health effects
- Low Concentrations: Irritation of the eyes, nose, and throat.
- High Concentrations: Severe respiratory distress, lung damage, and possible death.

Chlorine (Cl2)
Produced as a byproduct of industrial processes
(e.g., water treatment, bleach production).
Specifications we measure
| Range | Resolution | Technology | Sensor Module | |
| 50 PPM | 0.1 | Electrochemical | ES-CL2-4EC50 |
Guidelines
- OSHA:0.5 ppm over an 8-hour workday, with a 1 ppm STEL for 15 minutes
- US EPA: No specific standard set, but exposure limits typically range around 0.1 ppm in ambient air.
Health effects
- Low Concentrations: Eye, skin, and respiratory tract irritation.
- High Concentrations:Chemical burns, pulmonary edema, and death.

Hydrogen Chloride (HCl)
Hydrogen chloride is produced by the reaction of chlorine gas (Cl₂) with hydrogen (H₂) or by the combustion of chlorinated organic compounds
(e.g., PVC).
Specifications we measure
| Range | Resolution | Technology | Sensor Module | |
| 50 PPM | 0.5 | Electrochemical | ES-HCL-4EC50 |
Guidelines
- OSHA:5 ppm over an 8-hour workday.
- US EPA: No specific standard, but typically recommended to keep exposure levels under 0.1 ppm.
Health effects
- Low Concentrations: Irritation of the eyes, nose, and throat.
- High Concentrations:Severe respiratory issues, pulmonary edema, and long-term lung damage.

Bromine (Br2)
Bromine is produced through the reaction of chlorine with sodium bromide or from natural brine sources, such as seawater.
Specifications we measure
| Range | Resolution | Technology | Sensor Module | |
| 100 PPM | 1 | Electrochemical | ES-BR2-4EC100 |
Guidelines
- OSHA:0.1 ppm over an 8-hour workday.
- US EPA: No specific NAAQS, but recommended to limit exposure to no more than 0.003 ppm for ambient air
Health effects
- Low Concentrations: Eye and throat irritation, coughing
- High Concentrations:Severe respiratory distress, lung damage, chemical burns, and death.

Hydrogen Cyanide (HCN)
Produced in industrial processes such as electroplating, plastic manufacturing, and as a byproduct of combustion(e.g., from burning plastics).
Specifications we measure
| Range | Resolution | Technology | Sensor Module | |
| 50 PPM | 0.2 | Electrochemical | ES-HCN-4EC50 |
Guidelines
- OSHA:10 ppm over an 8-hour workday
- US EPA: No specific NAAQS for hydrogen cyanide, but typically around 0.2 ppb (parts per billion) for ambient air.
Health effects
- Low Concentrations: Headache, dizziness, nausea
- High Concentrations:Rapid respiratory and cardiac failure, loss of consciousness, and death..

Hydrogen Fluoride (HF)
Hydrogen fluoride is produced by the reaction of calcium fluoride (CaF₂) with sulfuric acid (H₂SO₄) or by the direct reaction of hydrogen and fluorine.
Specifications we measure
| Range | Resolution | Technology | Sensor Module | |
| 10 PPM | 0.1 | Electrochemical | ES-HF-4EC10 |
Guidelines
- OSHA:3 ppm over an 8-hour workday
- US EPA: No specific NAAQS for hydrogen fluoride, but recommended ambient concentration limits are typically set at 0.1 ppm.
Health effects
- Low Concentrations: Eye and skin irritation, sore throat, coughing.
- High Concentrations:Severe respiratory damage, pulmonary edema, and systemic toxicity affecting bones, liver, and kidneys.

Ethylene Oxide (EtO)
Ethylene oxide is produced by the reaction of ethylene with oxygen, usually in the presence of a silver catalyst at high temperatures
Specifications we measure
| Range | Resolution | Technology | Sensor Module | |
| 100 PPM | 0.1 | Electrochemical | ES-ETO-4EC20 |
Guidelines
- OSHA:3 ppm over an 8-hour workday
- US EPA: No specific NAAQS for hydrogen fluoride, but recommended ambient concentration limits are typically set at 0.1 ppm.
Health effects
- Short Term: 1 ppm over an 8-hour workday, with a 5 ppm STEL for 15 minutes
- Long Term:No specific NAAQS, but recommended exposure levels are based on cancer risk assessments, typically around 0.0003 ppm (for long-term exposure)

Phosgene (COCl2)
Phosgene is produced by the reaction of carbon monoxide (CO) with chlorine (Cl₂) at high temperatures or in the presence of a catalyst
Specifications we measure
| Range | Resolution | Technology | Sensor Module | |
| 10 PPM | 0.1 | Electrochemical | ES-COCL2-4EC10 |
Guidelines
- OSHA:3 ppm over an 8-hour workday
- US EPA: No specific NAAQS for hydrogen fluoride, but recommended ambient concentration limits are typically set at 0.1 ppm.
Health effects
- Low Concentrations: Irritation of the eyes and respiratory system, coughing, and nausea.
- High Concentrations: Severe respiratory distress, pulmonary edema (fluid in the lungs), and death.

Hydrogen Bromide (HBr)
Hydrogen bromide is produced by the reaction of bromine with hydrogen or by the reaction of hydrogen chloride with sodium bromide.
Specifications we measure
| Range | Resolution | Technology | Sensor Module | |
| 100 PPM | 1 | Electrochemical | ES-HBR-4EC20 |
Guidelines
- OSHA:3 ppm over an 8-hour workday
- US EPA: No specific NAAQS for hydrogen fluoride, but recommended ambient concentration limits are typically set at 0.1 ppm.
Health effects
- Low Concentrations: Irritation of the eyes, skin, and respiratory system
- High Concentrations: Severe respiratory distress, pulmonary edema, and long-term lung damage.

Hydrogen Peroxide (H2O2)
Commonly made via the anthraquinone process, where hydrogen and oxygen react in the presence of a catalyst.
Specifications we measure
| Range | Resolution | Technology | Sensor Module | |
| 500 PPM | 0.2 | Electrochemical | ES-HP-4EC500 |
Guidelines
- OSHA:1 ppm for 8-hour exposure
- NIOSH REL: 1 ppm (10-hour TWA).for airborne exposure.
Health effects
- Causes skin and eye irritation, respiratory issues, and toxicity if ingested or inhaled, leading to nausea, burns, or lung damage.

Carbon Disulfide (CS2)
Produced by reacting carbon with sulfur at high temperatures
Specifications we measure
| Range | Resolution | Technology | Sensor Module | |
| 100 PPM | 0.1 | Electrochemical | ES-CS2-4EC100 |
Guidelines
- OSHA:20 ppm as an 8-hour time-weighted average (TWA).
- NIOSH REL: 1 ppm (10-hour TWA).
Health effects
- Affects the nervous system, causing headaches, memory loss, and irritability. It also irritates the skin, eyes, and respiratory system.

Lower Explosive Limit (LEL)
LEL is not a substance but a property of various gases, indicating the lowest concentration that can ignite in air.
Specifications we measure
| Range | Resolution | Technology | Sensor Module | |
| 100 PPM | 0.1 | Catalytic Pallister | ES-LEL-4CP100 |
Guidelines
- OSHA recommends maintaining gas concentrations below the Lower Explosive Limit to prevent explosion hazards and ensure proper ventilation in the workspace. Regularly monitor gas levels using detectors to avoid risks associated with explosive concentrations.
Health Effects
- LEL is a measure of explosivity, not directly toxic. Exposure to gases near LEL can lead to explosion hazards, and in confined spaces, it could cause asphyxiation or injury if ignited.

C3H8 (Propane)
Propane is produced through natural gas processing and petroleum refining.
Specifications we measure
| Range | Resolution | Technology | Sensor Module | |
| 100 PPM | 0.1 | Catalytic Pallister | ES-LEL-4CP100 |
Guidelines
- OSHA recommends a permissible exposure limit (PEL) of 1,000 ppm for propane.
- Work areas should be well-ventilated, and gas concentrations should be continuously monitored.
- Propane storage should be handled according to safety guidelines to prevent leaks and minimize explosion risk.
Health effects
- Short-term: Inhalation at high concentrations can cause dizziness, nausea, headaches, and respiratory issues.
- Long-term: Prolonged exposure can lead to CNS depression and suffocation in confined spaces.
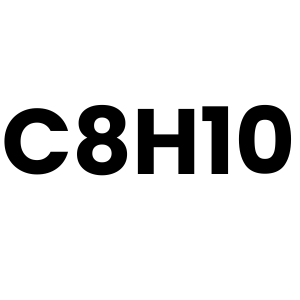
C8H10 (Toluene)
Toluene is produced as a byproduct of petroleum refining and natural gas processing.
Specifications we measure
| Range | Resolution | Technology | Sensor Module | |
| 100 PPM | 1 | Electrochemical | ES-XY-4EC100 |
Guidelines
- OSHA recommends a PEL of 200 ppm for toluene with a short-term exposure limit (STEL) of 300 ppm.
- Ensure proper storage, ventilation, and the use of personal protective equipment (PPE) to limit exposure.
Health effects
- Short-term: Toluene exposure can cause headaches, dizziness, CNS depression, and nausea.
- Long-term: Prolonged exposure can lead to liver/kidney damage, hearing loss, and neurological impairments.

LPG (Liquefied Petroleum Gas)
A mixture of propane and butane, LPG is produced during the refining of crude oil and natural gas.
Specifications we measure
| Range | Resolution | Technology | Sensor Module | |
| 100 %LEL | 1 | Catalytic Pallister | ES-COM-4CP100 |
Guidelines
- OSHA recommends a PEL of 1,000 ppm for propane and butane.
- Ensure proper ventilation, monitor concentrations, and store LPG in well-ventilated, secure areas to avoid accumulation and leakage, minimizing the risks of fire and explosions.
Health effects
- Short-term: Inhalation can cause headaches, dizziness, shortness of breath, and nausea. High exposure can lead to unconsciousness or suffocation.
- Long-term: Chronic exposure can cause CNS and respiratory issues.

NG (Natural Gas)
Natural gas is extracted from underground reserves, often via drilling or hydraulic fracturing (fracking).
Specifications we measure
| Range | Resolution | Technology | Sensor Module | |
| 100 PPM | 0.1 | Catalytic Pallister | ES-LEL-4CP100 |
Guidelines
- OSHA recommends using gas detectors in confined spaces and ensuring proper ventilation to keep natural gas concentrations below explosive limits.
- Follow local regulations for natural gas storage and emissions to avoid hazards.
Health effects
- Short-term: Methane displaces oxygen in the air, leading to dizziness, headaches, or suffocation in high concentrations.
- Long-term: No direct toxic effects from methane, but long-term exposure in confined spaces without proper ventilation can lead to asphyxiation.

CH4 (Methane)
A mixture of propane and butane, LPG is produced during the refining of crude oil and natural gas.
Specifications we measure
| Range | Resolution | Technology | Sensor Module | |
| 100 %LEL | 1 | Catalytic Pallister | ES-COM-4CP100 |
Guidelines
- LEL = 5%, UEL = 15%. Ensure ventilation, use gas detectors, and comply with methane emission regulations.
Health effects
- Short-term:Methane is non-toxic, but it can displace oxygen, leading to suffocation or dizziness in high concentrations.
- Long-term:No direct health effects, but it contributes to global warming as a potent greenhouse gas.

C4H10 (Butane)
Butane is produced during natural gas processing and as a byproduct of petroleum refining.
Specifications we measure
| Range | Resolution | Technology | Sensor Module | |
| 100 %LEL | 1 | Catalytic Pellistor | ES-COM-4CP100 |
Guidelines
- OSHA recommends a permissible exposure limit (PEL) of 800 ppm for butane. Work areas should be properly ventilated, and proper safety protocols must be followed for handling and storing butane to prevent accumulation and the risk of explosion.
Health effects
- Short-term: Inhalation can cause headaches, dizziness, shortness of breath, and nausea. High exposure can lead to unconsciousness or suffocation.
- Long-term: Chronic exposure can cause CNS and respiratory issues.

HC (Hydrocarbons)
Hydrocarbons are produced during the extraction, refining, and processing of petroleum and natural gas. They can be alkanes, alkenes, or aromatic compounds found in fuels like gasoline and diesel.
Specifications we measure
| Range | Resolution | Technology | Sensor Module | |
| 100 PPM | 0.1 | Catalytic Pellistor | ES-LEL-4CP100 |
Guidelines
- OSHA recommends controlling exposure to hydrocarbons by ensuring proper ventilation, using personal protective equipment (PPE),
- .Concentrations should be kept below the permissible exposure limits (PEL), and flammable hydrocarbons should be handled with caution to prevent fire hazards.
Health effects
- Short-term:Exposure to hydrocarbons can cause dizziness, headaches, nausea, and irritation of the eyes, skin, and respiratory system.
- Long-term:Chronic exposure can lead to liver and kidney damage, CNS depression, and increased risk of cancer. Prolonged exposure to high concentrations may also result in nerve damage.

asphyxiant (O2)
An asphyxiant is a substance that can displace oxygen in the air, leading to oxygen deficiency and potential harm to human health. Oxygen (O₂) is considered an asphyxiant when its concentration falls below normal levels (21%) due to the presence of other gases, such as nitrogen or carbon dioxide.
Oxygen deficiency can occur in confined spaces due to the use of inert gases (like nitrogen) in industrial processes, leaks, combustion, or when oxygen is consumed by biological processes (e.g., in sewage treatment).
Specifications we measure
| Range | Resolution | Technology | Sensor Module | |
| 25 | 0.1 | Electrochemical | ES-O2-4EC25 ES-O2-4EC25-LL |
Guidelines
- OSHA
19.5% (minimum oxygen concentration in the air). Below this level, workers may be at risk for asphyxiation.
Health effects
Prolonged exposure to oxygen-deficient environments can cause permanent brain and organ damage

Particulate Matter (PM)
PM10: Produced from construction, vehicular emissions, industrial processes, and natural sources like dust storms.
PM2.5: Forms from combustion, industrial emissions, and atmospheric chemical reactions.
PM1: Generated from combustion, industrial processes, and fine smoke particles.
Specifications we measure
| Range | Resolution | Technology | Sensor Module | |
| 0 - 1000 µg/m3 | 1 µg/m3 | Laser Scattering | ES-PM-ALS1000 |
Guidelines
- PM10 (EPA)
24-hour average: 150 µg/m³
Annual average: 50 µg/m³
- PM2.5 (EPA):
24-hour average: 35 µg/m³
Annual average: 12 µg/m³
Health effects
Respiratory issues (coughing, wheezing) , Aggravates asthma, bronchitis, and heart disease.
Long-term exposure:
Increases risk of cardiovascular diseases, stroke, lung cancer, and premature death ,PM2.5 and PM1 cause more severe health impacts due to their ability to penetrate deeper into the lungs.

VOC (Volatile Organic Compounds)
Produced from industrial emissions, vehicle exhaust, solvents, paints, and combustion processes.
Specifications we measure
| Range | Resolution | Technology | Sensor Module | |
| 2000 PPM | 1 | PID | ES-VOC-4PID2000 |
Guidelines
- OSHA doesn't set a specific PEL, but regulates individual VOCs
Health effects

CH2O (Formaldehyde)
Formed during combustion processes, vehicle emissions, industrial activities, and from building materials.
Specifications we measure
| Range | Resolution | Technology | Sensor Module | |
| 100 PPM | 0.5 | Electrochemical | ES-CH2O-4EC100 |
Guidelines
- OSHA PEL: 0.75 ppm (TWA)
- USEPA: Limits vary depending on the area and exposure.
Health effects

C2H4 (Ethylene)
Primarily produced from petrochemical processes and natural gas extraction.
Specifications we measure
| Range | Resolution | Technology | Sensor Module | |
| 2000 PPM | 1 | PID | ES-VOC-4PID2000 |
Guidelines
- No specific PEL, but controlled as part of VOC regulation.
Health effects
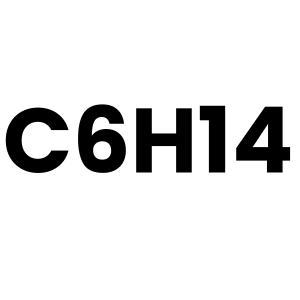
C6H14 (Hexane)
Emitted from petroleum refining, fuel combustion, and industrial processes.
Specifications we measure
| Range | Resolution | Technology | Sensor Module | |
| 2000 PPM | 1 | PID | ES-VOC-4PID2000 |
Guidelines
- OSHA PEL: 500 ppm (TWA)
- USEPA: Controlled as a hazardous air pollutant.
Health effects

C3H6O (Acetone)
Generated during industrial production, vehicle emissions, and the use of solvents.
Specifications we measure
| Range | Resolution | Technology | Sensor Module | |
| 2000 PPM | 1 | PID | ES-VOC-4PID2000 |
Guidelines
- OSHA PEL: 250 ppm (TWA)
- USEPA: Controlled for air quality.
